Even as you read this sentence, a neurochemical process has begun that will determine how long you remember it. And this process happens differently in men than in women, who are three times more likely to develop memory loss and Alzheimer’s disease as they age. Research indicates that a decline in estrogen is connected to memory loss, but scientists don’t have a complete understanding of how or why this happens.
Researcher Karyn Frick, a professor of psychology at the University of Wisconsin–Milwaukee (UWM), is working to understand the role of estrogen in memory loss and develop treatments for Alzheimer’s disease, which is the fifth-leading cause of death among Americans age 65 years and older.
Scientists already know that memory deficits are linked to a decline in estrogens, hormones found throughout the body that diminish during menopause. Estrogens are lipid molecules that act in the brain by binding to protein receptors on the surface of neurons, which in turn prompts chemical changes at the cellular level that mediate memory, attention, and mood, even addictive behaviors. Estrogens enhance brain functions in men, too, and testosterone is converted to estrogens in the male body for just this purpose.
Frick comes at the question of why memory problems arise more often in women than men as they age by watching mice at play. In her lab on the third floor of Garland Hall, Frick and her research team carefully document how well the mice navigate mazes, as well as what happens when they encounter a new toy or one that has been moved to an unexpected location. Over time, she and her research team have observed that the female middle-aged mice seemed “mentally old” compared to males of the same age, exhibiting symptoms of cognitive decline sooner than the males.
According to Frick, gender makes a big difference in how our brains age.
As with most diseases of the brain, the answer isn’t a simple one. Frick notes that doctors can’t just replace estrogens to prevent or reverse memory problems because, as with the mice, these hormones act differently on subjects who are different ages and who have differing levels of mental stimulation. While estrogens are needed to maintain bone density and healthy cholesterol levels, they are also linked to the incidence of depression and other mood disorders. Moreover, hormone replacement therapy for menopausal women can carry harmful side effects, such as an increased risk of breast cancer and cardiovascular problems.
To identify potential new drugs for dementia and Alzheimer’s disease—ones that don’t carry dangerous side effects and are equally effective for men and women—Frick must first unravel the intricate chain of cellular events through which estrogens enhance memory. To her, it’s a puzzle not unlike the mazes her mice must navigate to capture a prize.
During her undergraduate years at Franklin & Marshall College in Lancaster, Pennsylvania, Frick decided to pursue psychology. Then as now, her need to understand complexity was part of the appeal for researching how the brain works. Over time, Frick refined her focus to the brain’s hippocampus region, which is crucial for memory yet deteriorates with age and Alzheimer’s disease.
For me, the research is like detective work,” she says. “What’s really exciting is when you read something that triggers an I wonder what would happen if … moment.”
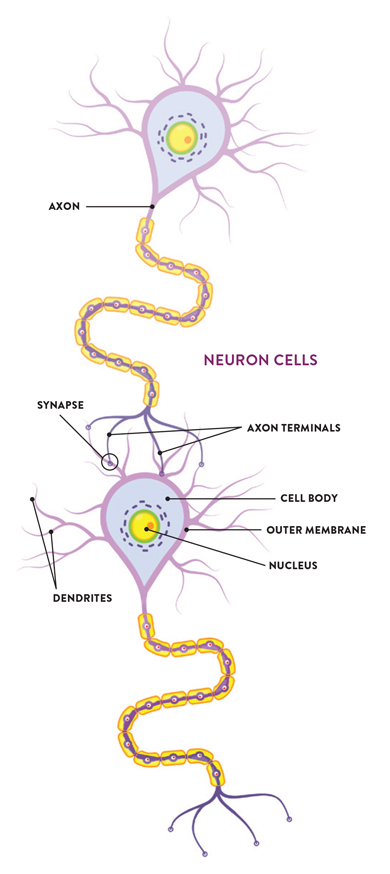 Memory starts and ends in the brain, where signals are sent along “relay stations” between two neurons, highly specialized cells that process and transmit intercellular signals. The axon, essentially the communication line for these signals, docks at either the outer membrane of the receiving neuron or one of its many dendrites, extensions that resemble tree branches protruding from the receiving neuron’s wall. Frick’s team studies what happens on the molecular level at these docking points.
Memory starts and ends in the brain, where signals are sent along “relay stations” between two neurons, highly specialized cells that process and transmit intercellular signals. The axon, essentially the communication line for these signals, docks at either the outer membrane of the receiving neuron or one of its many dendrites, extensions that resemble tree branches protruding from the receiving neuron’s wall. Frick’s team studies what happens on the molecular level at these docking points.
Chemicals called neurotransmitters direct brain communications when they bind to receptors found on neurons and their dendrites. Inside the receiving cell, neurotransmitters tell proteins to carry out genetic instructions, including memory-related tasks. Once it begins, the memory-related communication will happen with or without the estrogen. However, when estrogen is incorporated into the communication chain it amplifies the process, strengthening the memory created.
As estrogen production in the body winds down when menopause arrives, memory problems arise. “It’s not like menopausal women can’t remember anything,” notes Frick. “But with estrogens, they remember better.”
A few pieces of the puzzle came together in 2013, when Frick was the first researcher to link estrogens to one of the chemical processes known to make and strengthen memories. For estrogens to be effective, they must attach to specific receptors within both the dendrite and the outer membrane of the receiving neuron. Frick’s research team discovered that in order for the receptors in the two areas to carry estrogen’s message on to proteins inside the cell, a third receptor—for the neurotransmitter glutamate—must also be involved. The combination unleashes a cascade of cellular signaling that enables an enzyme, called extracellular signal-
regulated kinase, or ERK, to create a memory enhanced by estrogen.
Frick’s latest research has revealed some crucial differences in how this memory process work for women and for men. She’s discovered that, in women, the presence of estrogen links the hippocampus to a different part of the brain—the prefrontal cortex, where long-term memories are stored—and boosts memory mechanisms in both areas. Frick also has found that the activation of ERK in women occurs at the neuron’s outer membrane, where the receptors meet the dendrites. This is an important discovery, because proteins located within the cell’s outer membrane make for better targeting through drug delivery than those found inside the cell body.
But when Frick and UWM postdoctoral fellow Wendy Koss investigated the signaling chain in male mice infused with a potent form of estrogen, they got a surprise: Memory formation doesn’t happen through ERK activation as it does in females. “If the biochemical events leading to enhanced memory are different,” Frick says, “then you may need to develop drugs tailored to the mechanism specific for each gender.”
Armed with this new knowledge and in collaboration with partners at other universities, Frick is helping to kick-start the pharmacology for drug development by testing compounds that mimic the effects of estrogens but bind to a particular estrogen receptor that doesn’t induce negative health side effects. Early testing of one such compound indicated promising results in female mice.
One reason so little is known about the effects of estrogen in the body is that, for decades, researchers excluded females from studies in order to simplify their research. As a result, medical research is missing vital details on diseases and conditions—stroke, osteoporosis, and depression to name a few—that manifest differently in women and men.
Because of this disparity, the National Institutes of Health and the Federal Drug Administration have established new policies over the past twenty years intended to close the gender gap in medical research. Today, most medical studies include human subjects or animal models of both sexes.
While pleased with the progress in combating gender disparity in studies, Frick notes that interpretations of “mixed” male-female data are still questionable if the researchers don’t understand the individual endocrinology to begin with. “Researchers may say the behavior of models of both sexes is the same,” she says. “But it may be, as we are finding [with memory], that the biological process to get to the same end behavior is different. If you just looked at the behavioral level you’d miss them, upping the risk of more broadly sweeping conclusions.”
While noting the need for studies to take gender distinctions into account more carefully, Frick sees gender equity as the key to the success of the scientific endeavor. Through her teaching and mentorship, she’s inspired hundreds of students, particularly women and minorities, to pursue careers in neuroscience. Since joining the UWM faculty in 2010, Frick has hired mostly young women to help run her lab. She says she views her graduate students more as apprentices or colleagues than as students, regardless of their gender.
“It may be that deficiencies that exist in women’s health are attracting young women to the field,” she says. “I recently went to a conference of the Organization for the Study of Sex Differences, which included research on all aspects of health like gut microbiome, pain, and cardiovascular disease. About 80% of the attendees were women.”
Frick frequently speaks at conferences and symposia across the U.S., and she was recently named Investigator of the Year by the Alzheimer’s Association’s Southeastern Wisconsin Chapter. She’s also compiling and editing a book detailing the role of estrogens in cognitive functioning.
Frick’s work brings the wider conversation on women’s health to bear on the aging brain. “Few women are aware of the importance of talking about hormone replacement therapy with their physicians if they have a family history of Alzheimer’s,” she says. And yet, some of her research has shown that such therapy supports memory only if begun at the onset of menopause. Beginning the regimen later actually has detrimental effects on memory.
“It’s a powerful motivator for me and my students to know that our findings could ultimately help people,” Frick says. “Every finding allows us to see a new aspect of the puzzle and then put the pieces together.”
A version of this article first appeared in UWM Research magazine, 2018.